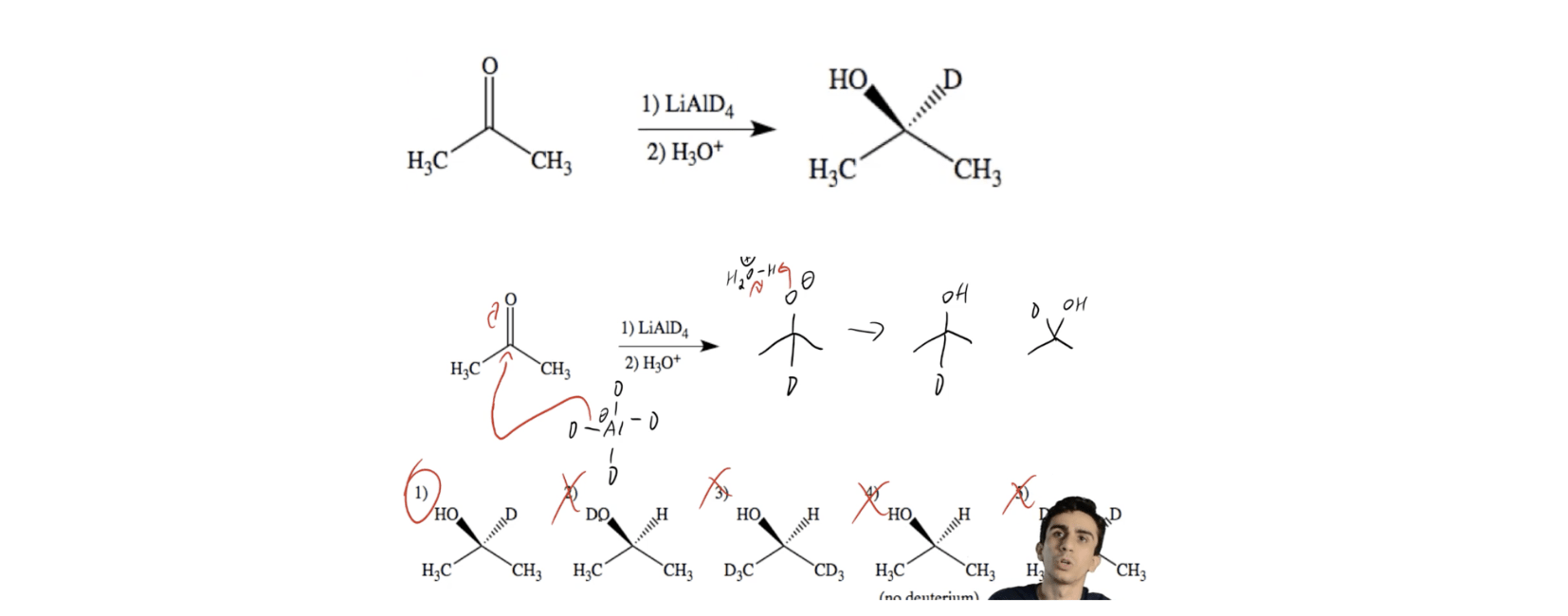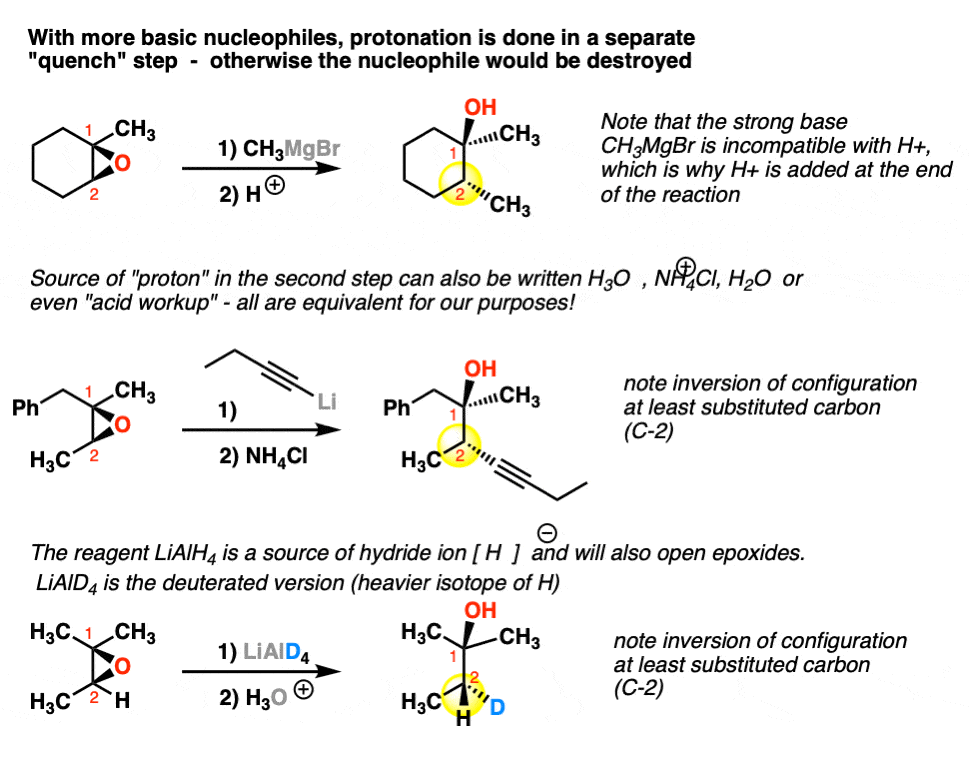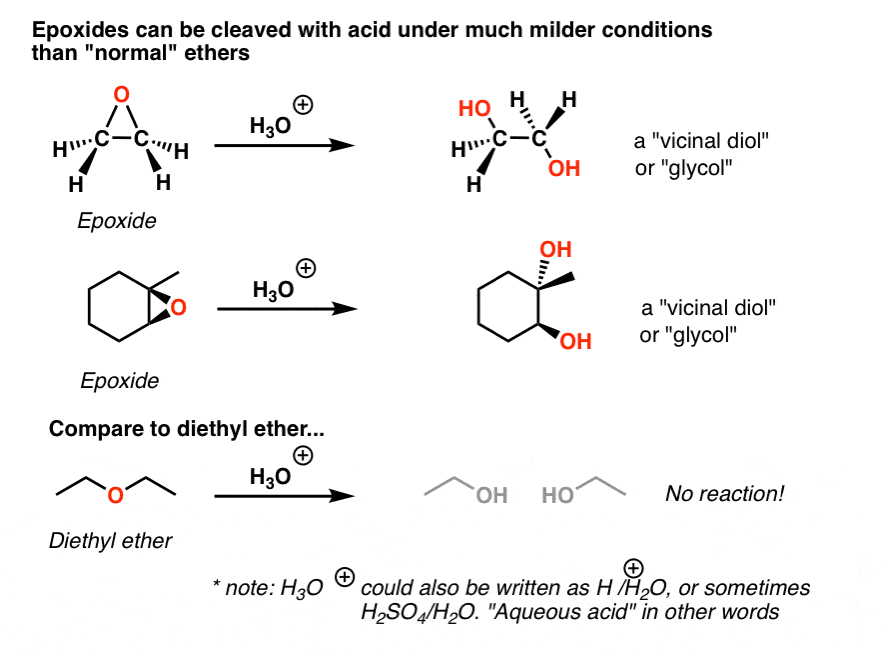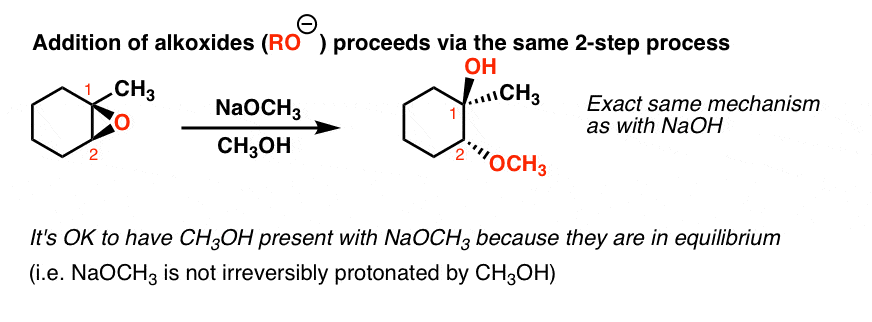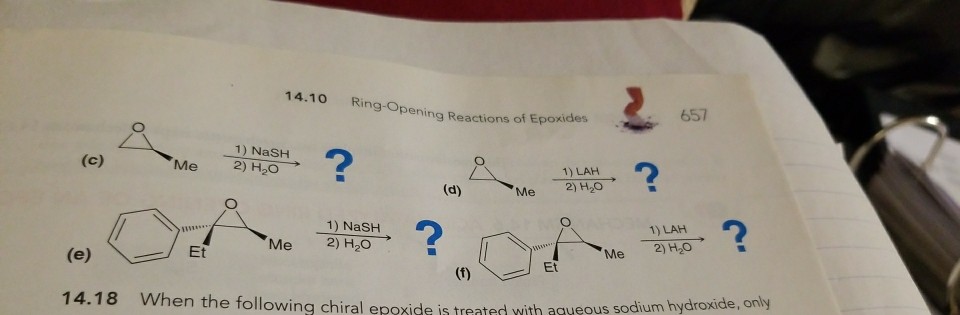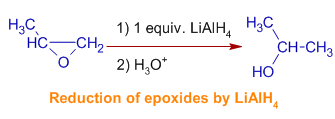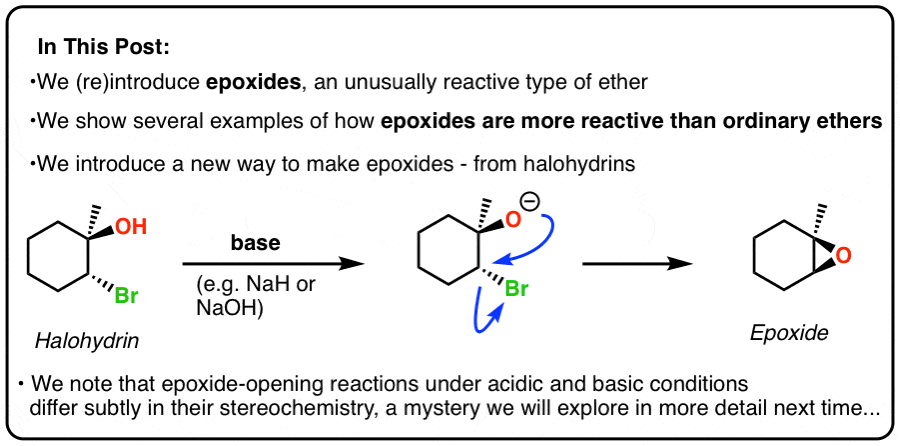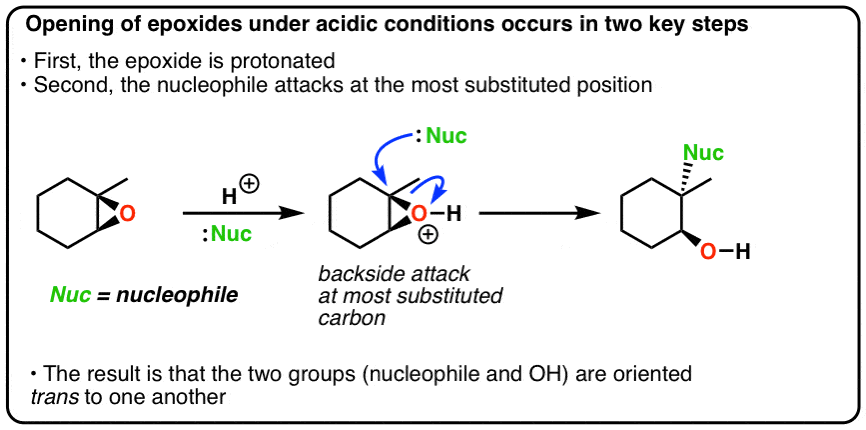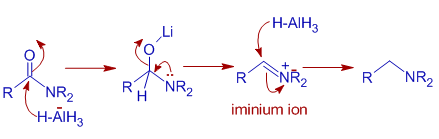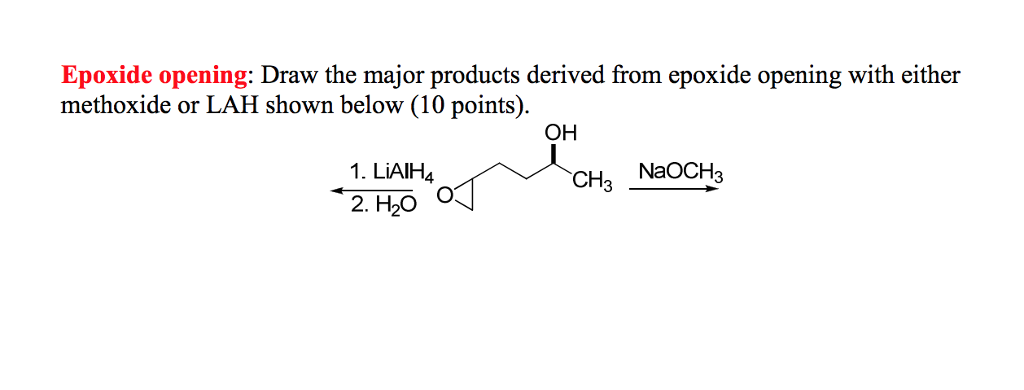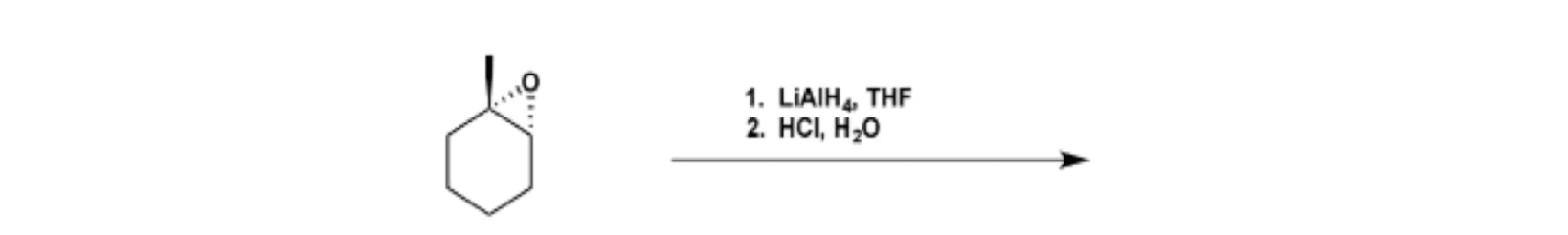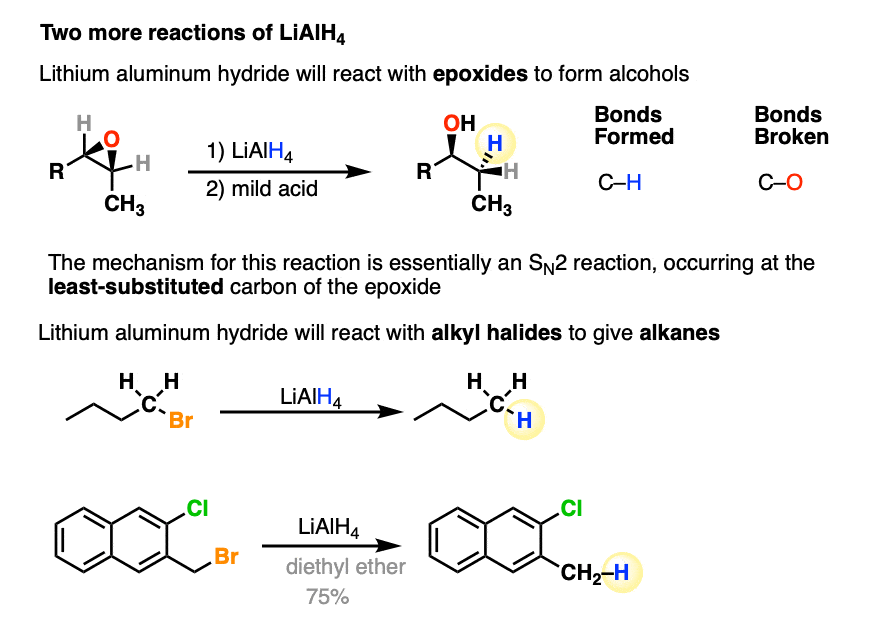
Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives – Master Organic Chemistry

Regiodivergent Hydroborative Ring Opening of Epoxides via Selective C–O Bond Activation | Journal of the American Chemical Society

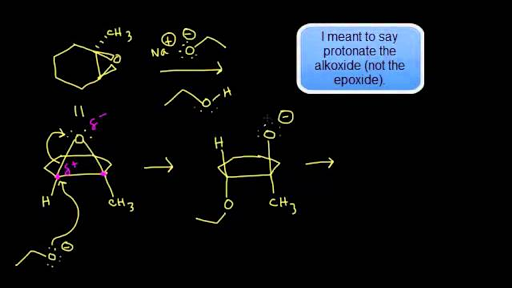
Epoxides are reduced by treatment with lithium aluminum hydride to yield alcohols. Propose a mechanism for this reaction. | Homework.Study.com
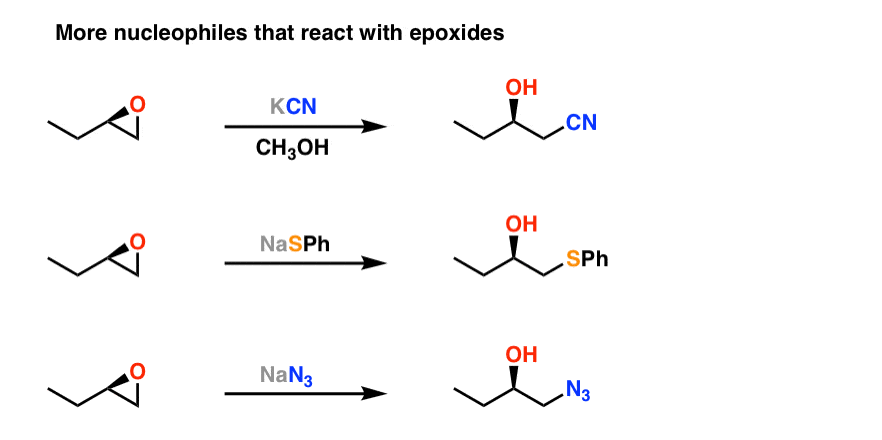
Reaction of epoxides with nucleophiles. (Adapted from 40.) Annu. Rev.... | Download Scientific Diagram

Regiodivergent Reductive Opening of Epoxides by Catalytic Hydrogenation Promoted by a (Cyclopentadienone)iron Complex | ACS Catalysis