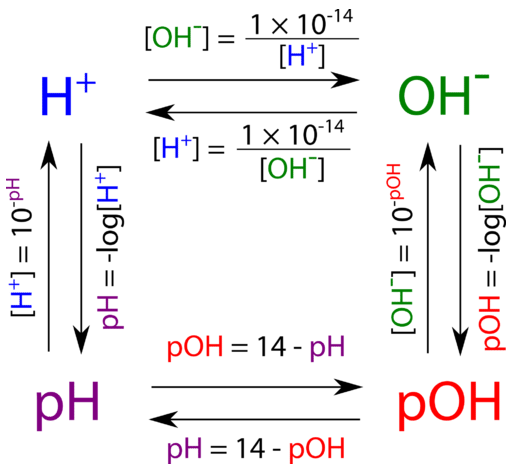
Stabilization of the tautomers HP(OH) 2 and P(OH) 3 of hypophosphorous and phosphorous acids as ligands - Dalton Transactions (RSC Publishing) DOI:10.1039/B510479C
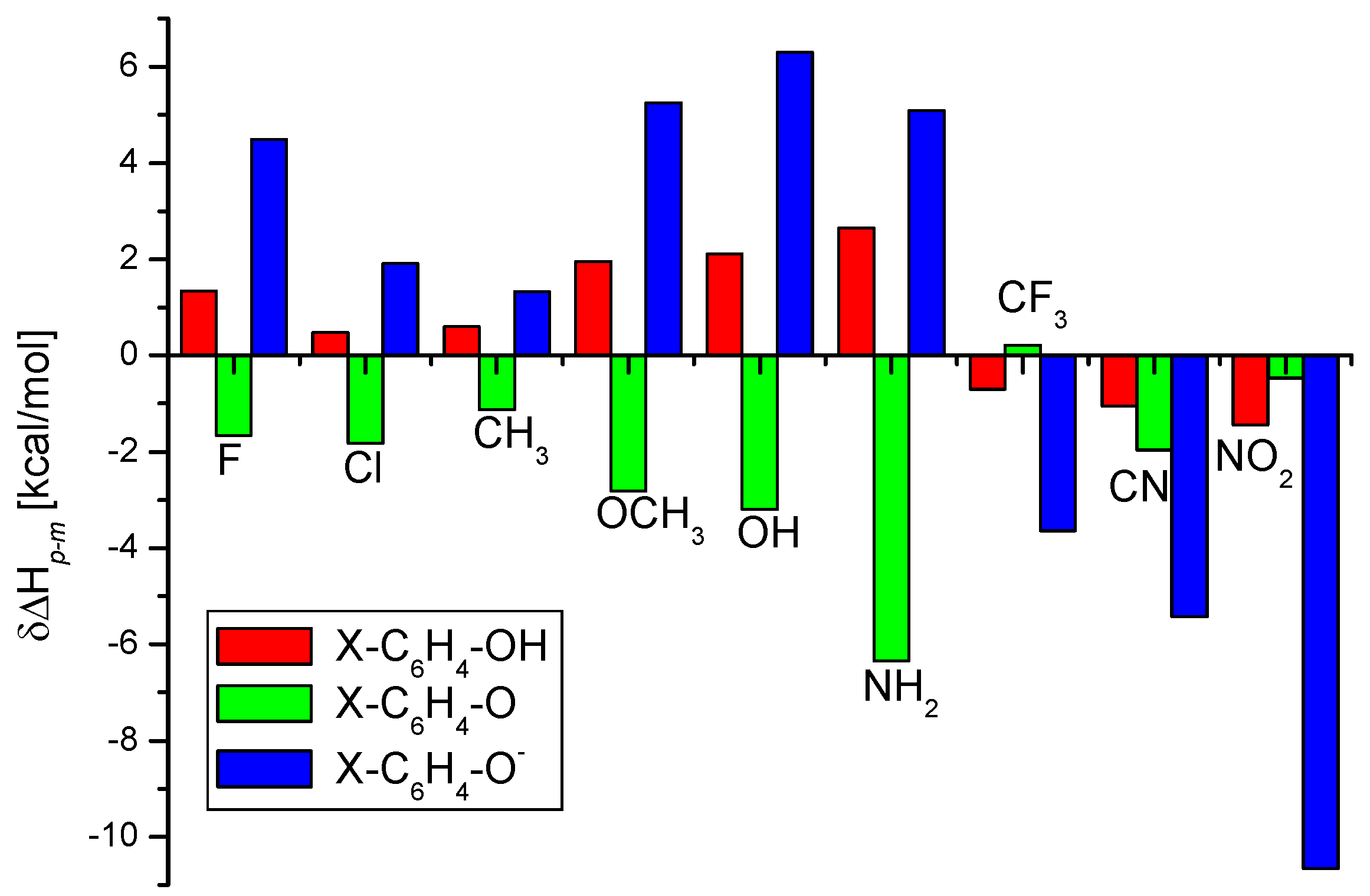
IJMS | Free Full-Text | The O-H Bond Dissociation Energies of Substituted Phenols and Proton Affinities of Substituted Phenoxide Ions: A DFT Study

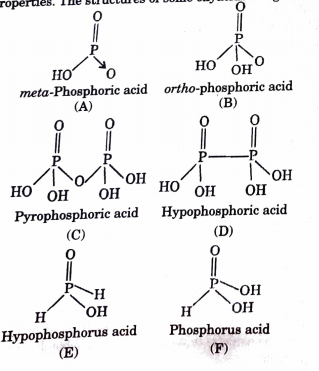
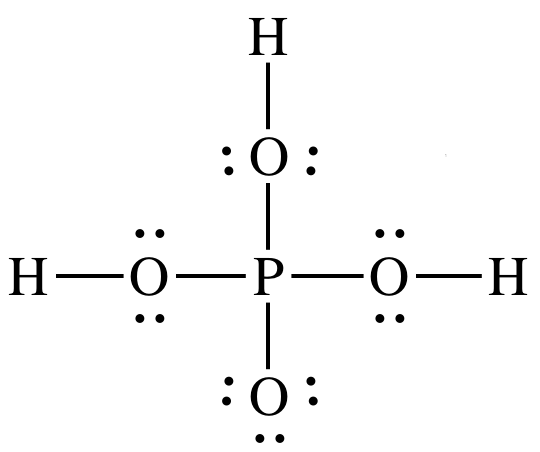
Phosphorous acid, H_3PO_3, has the structure (HO)_2PHO, in which one H atom is bonded to the P atom, and two H atoms are bonded to O atoms. For each bond to an
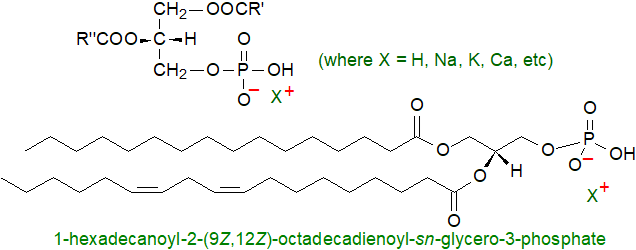
Phosphatidic acid, lysophosphatidic acid and the related lipids cyclic phosphatidic acid and pyrophosphatidic acid
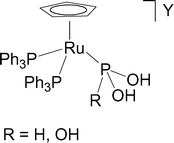
Stabilization of the tautomers HP(OH)2 and P(OH)3 of hypophosphorous and phosphorous acids as ligands - Dalton Transactions (RSC Publishing)

Why is phosphorous acid H3PO3 and not P(OH)3 - which should be more accurate as per the molecule structure? - Quora
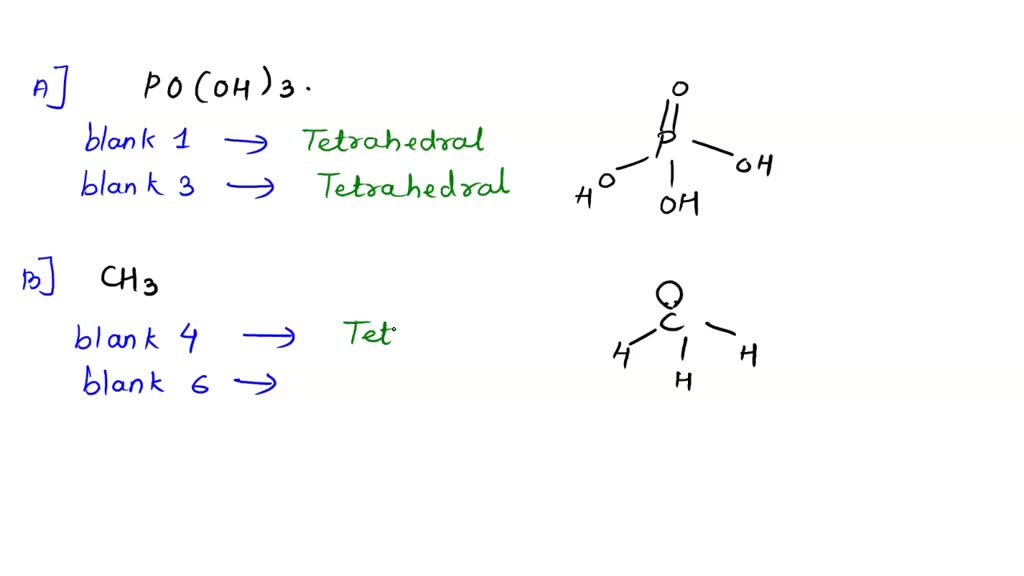
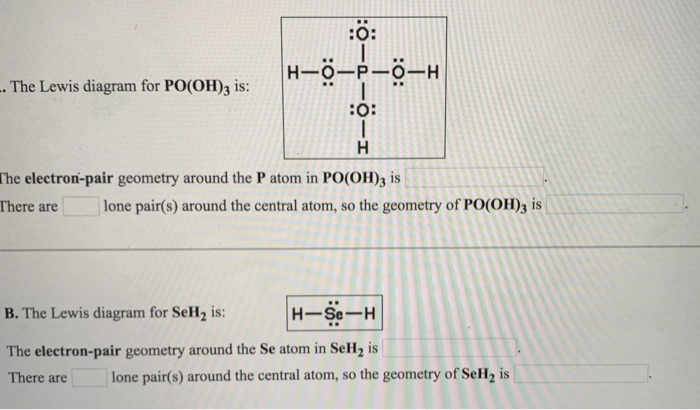
SOLVED: A. The Lewis diagram for PO(OH)3 is: The electron-pair geometry around the P atom in PO(OH)3 is fill in the blank 1. There are lone pair(s) around the central atom, so


![Q54E Phosphoric acid (H3PO4)is a t... [FREE SOLUTION] | StudySmarter Q54E Phosphoric acid (H3PO4)is a t... [FREE SOLUTION] | StudySmarter](https://studysmarter-mediafiles.s3.amazonaws.com/media/textbook-exercise-images/image_9jCIKPD.png?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIA4OLDUDE42UZHAIET%2F20230529%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20230529T000033Z&X-Amz-Expires=90000&X-Amz-SignedHeaders=host&X-Amz-Signature=53d3da6dfdf40bf065a0127fe3908055de6e981deda0a81acf97cb4f46b88b32)



![Solving For pH, pOH, [H+], [OH-] - Acids & Bases Solving For pH, pOH, [H+], [OH-] - Acids & Bases](http://youarebasic.weebly.com/uploads/5/0/1/4/50143245/5802360_orig.png)